Statutory Instruments
1992 No. 3162
PATENTS
The Patents (Supplementary Protection Certificate for Medicinal Products) Rules 1992
Laid before Parliament
11th December 1992
Coming into force
2nd January 1993
The Secretary of State, in exercise of the powers conferred by section 123 of, and paragraph 14 of Schedule 4 to, the Patents Act 1977(), of the power conferred upon him by the Department of Trade and Industry (Fees) Order 1988((), and of all other powers enabling him in that behalf, after consultation with the Council on Tribunals pursuant to section 8(1) of the Tribunals and Inquiries Act 1992() and with the consent of the Treasury pursuant to subsection (4) of the said section 123, hereby makes the following Rules:-
PART IGENERAL
Citation, commencement and extent
1.-(1) These Rules may be cited as the Patents (Supplementary Protection Certificate for Medicinal Products) Rules 1992 and shall come into force on 2nd January 1993.
(2) These Rules extend to Great Britain and Northern Ireland.
Interpretation
2.-(1) In these Rules-
"basic 1977 Act" means the Patents Act 1977;
"the patent" has the meaning assigned to it by paragraph (c) of Article 1 of the EC Regulation;
"certificate" has the meaning assigned to it in paragraph (d) of Article 1 of the EC Regulation;
"the comptroller" and "the journal" have the same meanings as they have in the 1977 Act;
"the court" has the same meaning as it has in the 1977 Act; and
"the EC Regulation" means Council Regulation (EEC) No. 1768/92 of 18th June 1992 concerning the creation of a supplementary protection certificate for medicinal products, the English language version of which is set out in Schedule 1 to these Rules(), and any reference in these Rules to an Article followed by a number is a reference to the Article so numbered in the EC Regulation; and
"register of patents" means the register of patents maintained pursuant to section 32 of the 1977 Act.
(2) Subject to paragraph (3), the forms of which the use is required by these Rules are those set out in Schedule 2 to these Rules.
(3) A requirement under these Rules to use such a form is satisfied by the use either of a replica of that form or of a form which is acceptable to the comptroller and contains the information required by the form set out in Schedule 2 to these Rules.
(4) The fees to be paid in respect of any matter arising under these Rules shall be those (if any) prescribed in relation to such matter in Schedule 4 to these Rules; and any reference to "prescribed fee" and "fees" in these Rules shall be construed accordingly.
PART IIPROVISIONS RELATING TO ARTICLES 4 TO 18 OF THE EC REGULATION
Application and fee in respect of application (Article 8 and 9(1))
3.-(1) The application for a certificate shall be-
(a)subject to the payment to the Patent Office of a prescribed fee; and
(b)lodged with the Patent Office accompanied by the prescribed fee.
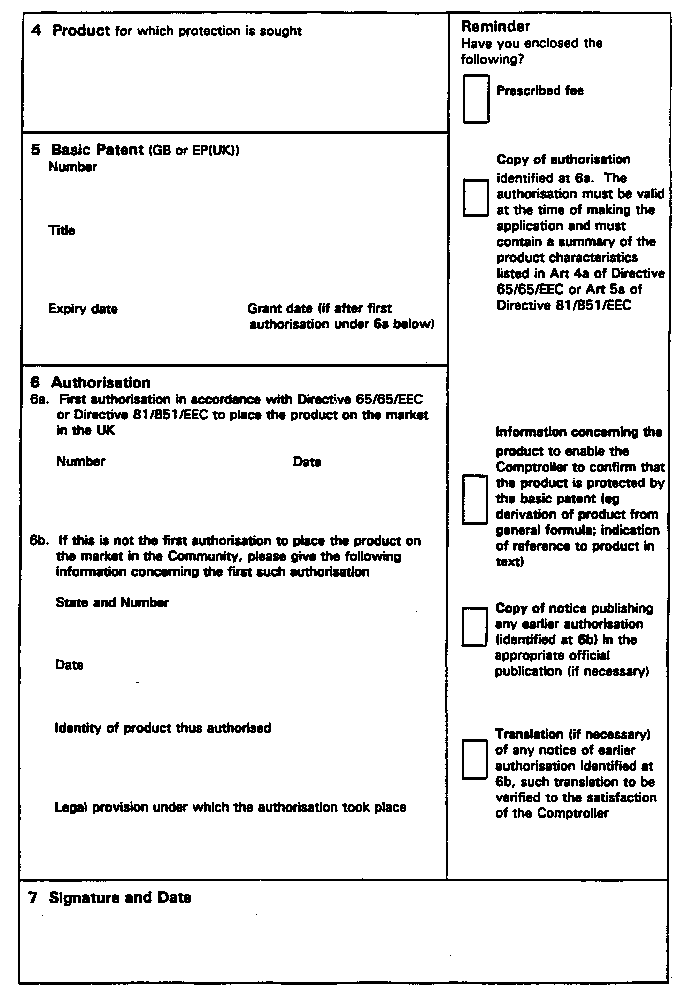
(2) A request for the grant of a certificate shall be made on Form SP1.
Certificate of grant (Article 10)
4. A certificate shall be in the form set out in Schedule 3 to these Rules.
Fees in respect of effective period of certificate (Article 12)
5.-(1) A reference in this rule to-
(a)"due date" means the date on which a certificate, subject to the requirement to pay fees, would take effect at the end of the lawful term of the basic patent; and
(b)"maximum period" means the maximum possible period of duration of a certificate as determined in accordance with Article 13.
(2) A certificate shall not take effect, and its actual duration shall not be determined, until payment is made of the fees prescribed in accordance with paragraphs (3) to (10) below.
(3) Subject to paragraph (9), the amount of fees payable in order for a certificate to take effect in respect of any period ("the appropriate fees") shall be the amount calculated by reference to the length of the maximum period, less, if any, such period deducted from the end of the maximum period during which it is desired by the holder of a certificate that the certificate shall not have effect, the resulting period, whether reduced from the maximum period or not, being referred to hereafter as the "effective period".
(4) The appropriate fees payable in respect of any effective period shall be the cumulative amount of fees prescribed-
(a)by reference to the successive twelve month periods of which an effective period is made up (any period of less than twelve months being treated as a twelve month period of which that lesser period forms part); the first such period shall commence on the due date ("the first year"); the second shall commence immediately upon expiry of the first ("the second year"), with corresponding provision in respect of each successive year up to a maximum of five years ("the fifth year") which years shall be referred to herein generally as "effective years", and
(b)in respect of each of the effective years, by the prescribed fees in force-
(i) where payment is made before the due date, on the date on which payment is made;
(ii)in any other case, on the due date.
(5) Subject to paragraphs (7) and (9), the appropriate fees in respect of an effective period shall be paid not later than the due date but may not be paid earlier than three months preceding the due date.
(6) Without prejudice to the provisions of paragraphs (2) and (5), the comptroller shall write to the holder of a certificate not later than two months before the due date-
(a)notifying him of the due date;
(b)indicating the prescribed fees applicable in respect of each of the effective years of which the maximum period of the certificate is made up; and
(c)specifying the period within which fees must be paid to the Patent Office in order for the certificate to take effect;
and the holder of the certificate shall, within the period specified under paragraph (c), notify the Patent Office on Form SP2 of the effective period of the certificate, which notification shall be accompanied by the appropriate fees payable in respect of that period.
(7) Where a certificate is granted later than three months before the end of the lawful term of the basic patent, the provisions of paragraphs (5) and (6) shall be modified as follows-
(a)the due date for the purposes of payment of the appropriate fees shall be the date three months after the date of grant of the certificate; and
(b)the comptroller shall write to the applicant for the certificate in the terms prescribed by paragraph (6), subject to subparagraph (a) of this paragraph, on the date on which he notifies him of the grant thereof.
(8) Where the effective period is less than the maximum period of the certificate it shall not subsequently be extended.
(9) Where the period for payment of fees under paragraph (5) or (7), as the case may be, has expired-
(a)the comptroller shall, not later than six weeks after the applicable due date and if the fees remain unpaid, notify the holder of the certificate-
(i)that the fees remain unpaid; and
(ii) of the consequences of non-payment; and
(iii)of the provisions of subparagraph (b);
(b)the holder, subject to the payment within a period of six months after the applicable due date of the unpaid fees and an additional late payment fee of an amount equal to one half of the amount of the unpaid fees, shall be treated as having paid the fees on the applicable due date.
(10) The notices under paragraphs (6) and (9) of this rule shall be sent by the comptroller to-
(a)the address for service furnished in writing by the applicant for a certificate or any address replacing it, and,
(b)in relation to the basic patent in respect of which the certificate is granted, where it differs from the address referred to in subparagraph (a),
(i)the address in the United Kingdom to which any renewal reminder is to be sent as specified by the proprietor on payment of the last renewal fee or any address replacing it, or
(ii)where no such address is specified, the address for service (if any) entered in the register of patents.
6. If the certificate is surrendered or declared invalid on or with effect from a date earlier ("the earlier date") than the date of expiry of the effective period, where the appropriate fees in respect of that period have been paid, the comptroller shall remit the fee paid in respect of any effective year which falls after the end of the effective year (if any) into which the earlier date falls.
Declaration of lapse or invalidity of certificate (Articles 14(d) and 15(1)(a) and (c))
7.-(1) On the application of any person, the comptroller may, as the case may be, declare-
(a)that a certificate has lapsed on the ground set out in Article 14(d); or
(b)that the ground for lapse under Article 14(d) no longer exists.
(2) The court or the comptroller may declare that a certificate is invalid in accordance with the provisions of Article 15.
(3) An application to the comptroller for a declaration under paragraph (1)(a) or paragraph (2) shall be made on Form SP3 and shall be accompanied by a copy thereof and a statement in duplicate setting out fully the facts upon which the applicant relies and the relief which he seeks.
(4) The comptroller shall send a copy of the application and the statement to the holder of the certificate.
(5) Within the period of two months beginning on the date on which such copies are sent to him, the holder of the certificate shall, if he wishes to contest the application, file a counter-statement in duplicate at the Patent Office setting out fully the grounds on which the application is contested; and the comptroller shall send a copy of the counter-statement to the applicant.
(6) No further statement or counter-statement shall be served by either party without the leave or direction of the comptroller.
(7) The comptroller may give such directions as he may think fit with regard to the subsequent procedure.
8. If it appears to the comptroller that a certificate has lapsed in accordance with Article 14(d) he may on his own initiative declare that the certificate has lapsed but shall not do so without giving the holder of the certificate notice of his intention to make such a declaration and affording him an opportunity to make representations within two months of the date of the notice.
Forms for use in connection with certificates and applications for certificates (Article 18(1))
9. Those forms of which use is required by any provision of the 1977 Act or any rules made thereunder in relation to patents or applications for patents, except where replaced by the forms set out in Schedule 2 to these Rules, shall also be used, with the necessary changes, in the corresponding circumstances in relation to certificates or applications for certificates.
Publication of: application, grant of certificate, rejection of application, declaration of lapse or of invalidity or of termination of grounds of lapse of certificate (Articles 9(2), 11(1) and (2) and 16)
10. Notification of-
(a)the application for a certificate;
(b)the fact that a certificate has been granted;
(c)the fact that the application for a certificate has been rejected;
(d) lapse of a certificate;
(e)invalidity of a certificate;
(f)termination of grounds for lapse of a certificate under Article 14(d),
shall be published by the comptroller in the journal.
E. Leigh
Parliamentary Under Secretary of State for Trade and Technology
Department of Trade and Industry
10th December 1992
We consent to the making of these Rules.
Patrick Irvine
Tim Wood
Two of the Lords Commissioners of Her Majesty's Treasury
11th December 1992
Rule 2(1)
SCHEDULE 1COUNCIL REGULATION (EEC) NO. 1768/92 OF 18TH JUNE 1992 CONCERNING THE CREATION OF A SUPPLEMENTARY PROTECTION CERTIFICATE FOR MEDICINAL PRODUCTS.
THE COUNCIL OF THE EUROPEAN COMMUNITIES
Having regard to the Treaty establishing the European Economic Community, and in particular Article 100a thereof,
Having regard to the proposal from the Commission().
In cooperation with the European Parliament().
Having regard to the opinion of the Economic and Social Committee().
Whereas pharmaceutical research plays a decisive role in the continuing improvement in public health;
Whereas medicinal products, especially those that are the result of long, costly research will not continue to be developed in the Community and in Europe unless they are covered by favourable rules that provide for sufficient protection to encourage such research;
Whereas at the moment the period that elapses between the filing of an application for a patent for a new medicinal product and authorization to place the medicinal product on the market makes the period of effective protection under the patent insufficient to cover the investment put into the research;
Whereas this situation leads to a lack of protection which penalizes pharmaceutical research;
Whereas the current situation is creating the risk of research centres situated in the Member States relocating to countries that already offer greater protection;
Whereas a uniform solution at Community level should be provided for, thereby preventing the heterogeneous development of national laws leading to further disparities which would be likely to create obstacles to the free movement of medicinal products within the Community and thus directly affect the establishment and the functioning of the internal market;
Whereas, therefore, the creation of a supplementary protection certificate granted, under the same conditions, by each of the Member States at the request of the holder of a national or European patent relating to a medicinal product for which marketing authorization has been granted is necessary; whereas a Regulation is therefore the most appropriate legal instrument;
Whereas the duration of the protection granted by the certificate should be such as to provide adequate effective protection; whereas, for this purpose, the holder of both a patent and a certificate should be able to enjoy an overall maximum of fifteen years of exclusivity from the time the medicinal product in question first obtains authorization to be placed on the market in the Community;
Whereas all the interests at stake, including those of public health, in a sector as complex and sensitive as the pharmaceutical sector must nevertheless be taken into account, whereas, for this purpose, the certificate cannot be granted for a period exceeding five years; whereas the protection granted should furthermore be strictly confined to the product which obtained authorization to be placed on the market as a medicinal product;
Whereas a fair balance should also be struck with regard to the determination of the transitional arrangements; whereas such arrangements should enable the Community pharmaceutical industry to catch up to some extent with its main competitors who, for a number of years, have been covered by laws guaranteeing them more adequate protection, while making sure that the arrangements do not compromise the achievement of other legitimate objectives concerning the health policies pursued both at national and Community level;
Whereas the transitional arrangements applicable to applications for certificates filed and to certificates granted under national legislation prior to the entry into force of this Regulation should be defined;
Whereas special arrangements should be allowed in Member States whose laws introduced the patentability of pharmaceutical products only very recently;
Whereas provision should be made for appropriate limitation of the duration of the certificate in the special case where a patent term has already been extended under a specific national law, HAS ADOPTED THIS REGULATION:
Article 1
Definitions
For the purpose of this Regulation:
(a)"medicinal product" means any substance or combination of substances presented for treating or preventing disease in human beings or animals and any substance or combination of substances which may be administered to human beings or animals with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in humans or in animals;
(b)"product" means the active ingredient or combination of active ingredients of a medicinal product;
(c)"basis patent" means a patent which protects a product as defined in (b) as such, a process to obtain a product or an application of a product, and which is designated by its holder for the purpose of the procedure for grant of a certificate.
(d)"certificate" means the supplementary protection certificate.
Article 2
Scope
Any product protected by a patent in the territory of a Member State and subject, prior to being placed on the market as a medicinal product, to an administrative authorization procedure as laid down in Council Directive 65/65/EEC() or Directive 81/851/EEC() may, under the terms and conditions provided for in this Regulation, be the subject of a certificate.
Article 3
Conditions for obtaining a certificate
A certificate shall be granted if, in the Member State in which the application referred to in Article 7 is submitted and at the date of that application;
(a)the product is protected by a basic patent in force;
(b)a valid authorization to place the product on the market as a medicinal product has been granted in accordance with Directive 65/65/EEC or Directive 81/851/EEC, as appropriate;
(c) the product has not already been the subject of a certificate;
(d)the authorization referred to in (b) is the first authorization to place the product on the market as a medicinal product.
Article 4
Subject-matter of protection
Within the limits of the protection conferred by the basic patent, the protection conferred by a certificate shall extend only to the product covered by the authorization to place the corresponding medicinal product on the market and for any use of the product as a medicinal product that has been authorized before the expiry of the certificate.
Article 5
Effects of the certificate
Subject to the provisions of Article 4, the certificate shall confer the same rights as conferred by the basic patent and shall be subject to the same limitations and the same obligations.
Article 6
Entitlement to the certificate
The certificate shall be granted to the holder of the basic patent or his successor in title.
Article 7
Application for a certificate
1. The application for a certificate shall be lodged within six months of the date on which the authorization referred to in Article 3(b) to place the product on the market as a medicinal product was granted.
2. Notwithstanding paragraph 1, where the authorization to place the product on the market is granted before the basic patent is granted, the application for a certificate shall be lodged within six months of the date on which the patent is granted.
Article 8
Content of the application for a certificate
1. The application for a certificate shall contain:
(a)a request for the grant of a certificate stating in particular:
(i)the name and address of the applicant;
(ii) if he has appointed a representative, the name and address of the representative;
(iii)the number of the basic patent and the title of the invention;
(iv)the number and date of the first authorization to place the product on the market, as referred to in Article 3(b) and, if this authorization is not the first authorization for placing the product on the market in the Community, the number and date of that authorization;
(b)a copy of the authorization to place the product on the market, as referred to in Article 3(b), in which the product is identified, containing in particular the number and date of the authorization and the summary of the product characteristics listed in Article 4a of Directive 65/65/EEC or Article 5a of Directive 81/851/EEC;
(c)if the authorization referred to in (b) is not the first authorization for placing the product on the market as a medicinal product in the Community, information regarding the identity of the product thus authorised and the legal provision under which the authorization procedure took place, together with a copy of the notice publishing the authorization in the appropriate official publication.
2. Member States may provide that a fee is to be payable upon application for a certificate.
Article 9
Lodging of an application for a certificate
1. The application for a certificate shall be lodged with the competent industrial property office of the Member State which granted the basic patent or on whose behalf it was granted and in which the authorization referred to in Article 3(b) to place the product on the market was obtained, unless the Member State designates another authority for the purpose.
2. Notification of the application for a certificate shall be published by the authority referred to in paragraph 1. The notification shall contain at least the following information:
(a) the name and address of the applicant;
(b) the number of the basic patent;
(c)the title of the invention;
(d)the number and date of the authorization to place the product on the market, referred to in Article 3(b), and the product identified in that authorization;
(e)where relevant, the number and date of the first authorization to place the product on the market in the Community.
Article 10
Grant of the certificate or rejection of the application
1. Where the application for a certificate and the product to which it relates meet the conditions laid down in this Regulation, the authority referred to in Article 9(1) shall grant the certificate.
2. The authority referred to in Article 9(1) shall, subject to paragraph 3, reject the application for a certificate if the application or the product to which it relates does not meet the conditions laid down in this Regulation.
3. Where the application for a certificate does not meet the conditions laid down in Article 8, the authority referred to in Article 9(1) shall ask the applicant to rectify the irregularity, or to settle the fee, within a stated time.
4. If the irregularity is not rectified or the fee is not settled under paragraph 3 within the stated time, the authority shall reject the application.
5. Member States may provide that the authority referred to in Article 9(1) is to grant certificates without verifying that the conditions laid down in Article 3(c) and (d) are met.
Article 11
Publication
1. Notification of the fact that a certificate has been granted shall be published by the authority referred to in Article 9(1). The notification shall contain at least the following information:
(a)the name and address of the holder of the certificate;
(b) the number of the basic patent;
(c) the title of the invention;
(d)the number and date of the authorization to place the product on the market referred to in Article 3(b) and the product identified in that authorization;
(e)where relevant, the number and date of the first authorization to place the product on the market in the Community;
(f) the duration of the certificate.
2. Notification of the fact that the application for a certificate has been rejected shall be published by the authority referred to in Article 9(1). The notification shall contain at least the information listed in Article 9(2).
Article 12
Annual fees
Member States may require that the certificate be subject to the payment of annual fees.
Article 13
Duration of the certificate
1. The certificate shall take effect at the end of the lawful term of the basic patent for a period equal to the period which elapsed between the date on which the application for a basic patent was lodged and the date of the first authorization to place the product on the market in the Community reduced by a period of five years.
2. Notwithstanding paragraph 1, the duration of the certificate may not exceed five years from the date on which it takes effect.
Article 14
Expiry of the certificate
The certificate shall lapse:
(a) at the end of the period provided for in Article 13;
(b) if the certificate-holder surrenders it;
(c) if the annual fee laid down in accordance with Article 12 is not paid in time;
(d)if and as long as the product covered by the certificate may no longer be placed on the market following the withdrawal of the appropriate authorisation or authorizations to place on the market in accordance with Directive 65/65/EEC or Directive 81/851/EEC. The authority referred to in Article 9(1) may decide on the lapse of the certificate either of its own motion or at the request of a third party.
Article 15
Invalidity of the certificate
1. The certificate shall be invalid if:
(a)it was granted contrary to the provisions of Article 3;
(b)the basic patent has lapsed before its lawful term expires;
(c)the basic patent is revoked or limited to the extent that the product for which the certificate was granted would no longer be protected by the claims of the basic patent or, after the basic patent has expired, grounds for revocation exist which would have justified such revocation or limitation.
2. Any person may submit an application or bring an action for a declaration of invalidity of the certificate before the body responsible under national law for the revocation of the corresponding basic patent.
Article 16
Notification of lapse or invalidity
If the certificate lapses in accordance with Article 14(b), (c) or (d) or is invalid in accordance with Article 15, notification thereof shall be published by the authority referred to in Article 9(1).
Article 17
Appeals
The decisions of the authority referred to in Article 9(1) or of the body referred to in Article 15(2) taken under this Regulation shall be open to the same appeals as those provided for in national law against similar decisions taken in respect of national patents.
Article 18
Procedure
1. In the absence of procedural provisions in this Regulation, the procedural provisions applicable under national law to the corresponding basic patent shall apply to the certificate, unless that law lays down special procedural provisions for certificates.
2. Notwithstanding paragraph 1, the procedure for opposition to the granting of a certificate shall be excluded.
Article 19
Transitional provisions
1. Any product which, on the date on which this Regulation enters into force, is protected by a valid basic patent and for which the first authorization to place it on the market as a medicinal product in the Community was obtained after 1 January 1985 may be granted a certificate.
In the case of certificates to be granted in Denmark and in Germany, the date of 1 January 1985 shall be replaced by that of 1 January 1988.
In the case of certificates to be granted in Belgium and in Italy, the date of 1 January 1985 shall be replaced by that of 1 January 1982.
2. An application for a certificate as referred to in paragraph 1 shall be submitted within six months of the date on which this Regulation enters into force.
Article 20
This Regulation shall not apply to certificates granted in accordance with the national legislation of a Member State before the date on which this Regulation enters into force or to applications for a certificate filed in accordance with that legislation before the date of publication of this Regulation in the Official Journal of the European Communities.
Article 21
In those Member States whose national law did not on 1 January 1990 provide for the patentability of pharmaceutical products, this Regulation shall apply five years after the entry into force of this Regulation.
Article 19 shall not apply in those Member States.
Article 22
Where a certificate is granted for a product protected by a patent which, before the date on which this Regulation enters into force, has had its term extended or for which such extension was applied for, under national patent law, the term of protection to be afforded under this certificate shall be reduced by the number of years by which the term of the patent exceeds 20 years.
FINAL PROVISION
Article 23
Entry into force
This Regulation shall enter into force six months after its publication in the Official Journal of the European Communities.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Rule 2(3)
SCHEDULE 2GENERAL FORMS
Rule 4
SCHEDULE 3CERTIFICATE
Rule 2 (4)
SCHEDULE 4FEES
| Number of corresponding Supplementary Protection Certificate Form | Item | Amount |
|---|
| | £ |
|---|
| SP1 | Request for grant of certificate under Article 8 and rule 3 ... ... ... ... | 250 |
| SP2 | On payment of annual fees (and additional fee for late payment) under Article 12 and rule 5: |
| -for first year or part thereof ... ... ... ... | 600 |
| -for second year or part thereof ... ... ... ... | 700 |
| -for third year or part thereof ... ... ... ... | 800 |
| -for fourth year or part thereof ... ... ... ... | 900 |
| -for fifth year or part thereof ... ... ... ... | 1,000 |
| SP3 | Application for declaration of lapse or invalidity under Articles 14 and 15 ... ... ... ... | - |
For the Council
Vitor MARTINS
The President
Done at Luxembourg, 18 June 1992.
Explanatory Note
Council Regulation (EEC) No. 1768/92 ("the EC Regulation") (which is set out in Schedule 1 to these Rules) and which will take effect on 2nd January 1993, creates and sets out the conditions relating to applications for, and the grant of, a supplementary protection certificate for medicinal products ("the certificate"). The certificate, when granted, will extend the protection afforded by a patent in respect of a pharmaceutical product covered by it for a period which will not extend to a period more than five years from the date it takes effect.
The EC Regulation is directly applicable law in the United Kingdom and has effect so that the certificate confers the same rights and is subject to the same limitations and the same obligations as the basic patent; the decisions of the comptroller taken in respect of the certificate are open to the same appeals as those provided against similar decisions taken in respect of patents and, in the absence of specific procedural provisions in the EC Regulation or national laws, the procedural provisions applicable to the corresponding basic patent are to apply to the certificate.
These Rules are therefore necessary for implementation in part of the Community obligation to the extent that certain provisions of the EC Regulation enable the member States to make provision for the procedure applicable to the introduction of the certificate (in so far as that procedure is to differ from the procedure applicable to patents and applications for patents) (Article 18) and for the payment and the amount of fees (Articles 8(2) and 12).